This week, the Journal of Biological Chemistrypublished two side-by-side papers, which revealed the function and regulatory mechanisms of Calmodulin acetylation in the mouse brain. In view of the exceptional contribution to the field, these two papers have been selected as one of JBC's "Editors' Picks", as introduced by the authors from East China Normal University.
Calcium (Ca2+) signal is essential for almost all cellular processes. As an indicator of neural activity,Ca2+signal plays important roles in the processes of axon guidance, synaptic plasticity, learning and memory. Calmodulin (CaM) is a ubiquitousCa2+sensor and has more than 300 target proteins including protein kinases, enzymes, cytoskeleton proteins, ion and water channels. CaM plays critical roles in synaptic plasticity such as long-term potentiation (LTP). During LTP induction, activation of NMDA receptor triggersCa2+influx, and theCa2+binds with CaM and activatesCa2+/CaM-dependent protein kinase IIa(CaMKIIa).
Dong-Min Yin's group at the Key Laboratory of Brain Functional Genomics of the Ministry of Education, School of Life Sciences, East China Normal University, studies the function of protein acetylation in synaptic plasticity and learning. Previous studies in this area manily focused on histone protein acetylation in the nucleus. By analyzing the results from the public databases and previous proteomics studies, the researchers found that the acetylation of CaM is very rich in the mammalian brain.
The researchers generated antibodies against acetylated CaM and found that neural activity rapidly (within 1 minute) increased the level of CaM acetylation, and this process depends on NMDA receptors. Next, the researchers calculated the stoichiometry of CaM acetylation in the hippocampal total lysates, and found the percentage of acetylated CaM after neuronal stimulation is about 6%-7%, which is close to the level of histone protein acetylation. Moreover, mutation of acetyllysines in CaM1 proteins disrupts synaptic plasticity and fear learning in a mouse model. The authors further demonstrate that acetylation of CaM reduces the binding free energy and increases binding affinity towardCaMKIIa,a protein kinase pivotal to synaptic plasticity and learning.
This paper entitled "Acetylation of Calmodulin Regulates Synaptic Plasticity and Fear Learning" is published on line in theJournal of Biological Chemistry. Drs Hai-Long Zhang, Bing Zhao, and PhD student Han Wei are the co-first authors of thepaper, and professor Dong-Min Yin is the corresponding author of the paper. Professor Jian Zhang from Shanghai Jiaotong University provided support in the calculation of molecular dynamics.

Since acetylation of CaM plays an important role in synaptic plasticity and learning, then how is acetylation of CaM regulated by neural activity? To answer this question, the researches must identify the lysine acetyltransferase (KAT) responsible for acetylation of CaM. Toward this goal, the researchers overexpressed various KAT proteins in HEK293 cells, and found that Steroid Receptor Coactivator 3 (SRC3) was most potent to acetylate CaM.
The authors further demonstrate that SRC3 interacts with and acetylates CaM in a Ca2+and NMDA receptor-dependent manner. They also show that pharmacological inhibition or genetic downregulation of SRC3 impairs CaM acetylation, synaptic plasticity, and contextual fear learning in mice. Moreover, the effects of SRC3 inhibition on synaptic plasticity and fear learning could be rescued by 3KQ-CaM, a mutant form of CaM which mimics acetylation. Together, these observations demonstrate that SRC3 acetylates CaM to regulate synaptic plasticity and memory in mice (Figure 1).
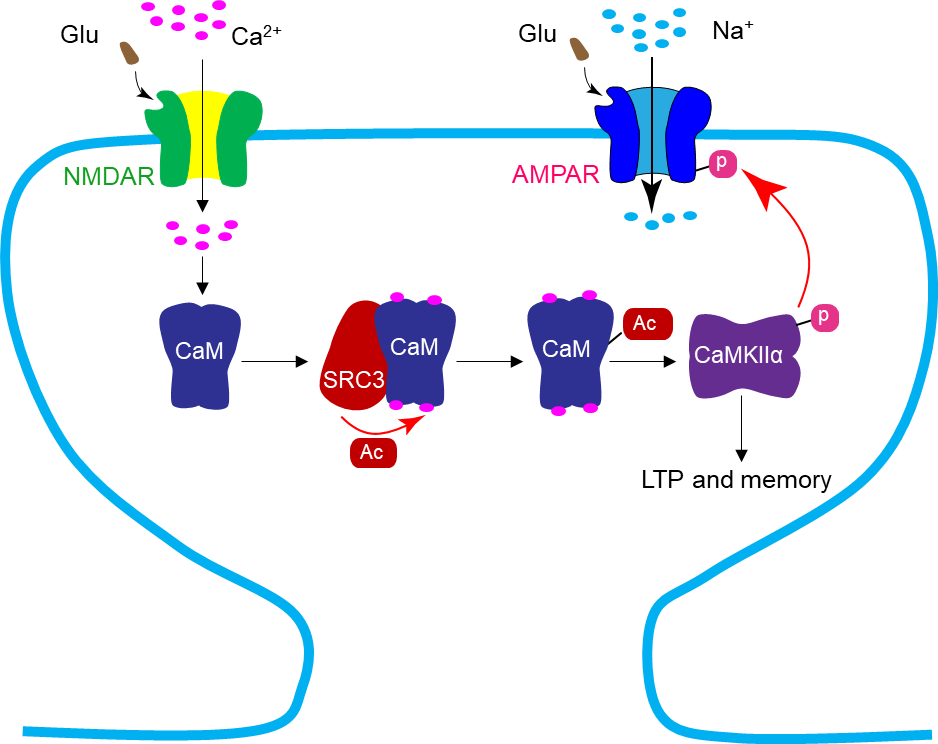 F
F
Figure 1 SRC3 acetylates CaM to regulate synaptic plasticity and memory. Neural activity activates NMDA receptors to cause Ca2+influx, which promotes acetylation of CaM by SRC3. The acetylated CaM is more readily to activate CaMKIIα, thereby regulating hippocampal long-term potentiation (LTP) and memory.
The second paper entitled"SRC3 Acetylates Calmodulin in the Mouse Brain to Regulate Synaptic Plasticity and Fear Learning", was published online in theJournal of Biological Chemistry. Dr. Hai-Long Zhang (currently a postdoctoral fellow at Soochow University), PhD student Wei Han, and master student Yin-Quan Du are the co-first authors of the paper, and professor Dong-Min Yin is the corresponding author of the paper (Figure 2). These two papers revealed the function and regulatory mechanisms of CaM acetylation, which may bring new perspectives to the study of Ca2+signal transduction.


Figure 2 Professor Dong-Min Yin (middle) and the first authors Dr. Hai-Long Zhang(now working at Soochow University), PhD student Wei Han (right), and master student Yin-Quan Du (left).
Dong-Min Yin's group studies the mechanisms of synaptic plasticity and schizophrenia. This work was funded by the National Natural Science Foundation of China, Shanghai Eastern Scholars, the State Key Laboratory of Neuroscience of the Chinese Academy of Sciences, and the Shanghai Key Laboratory of Severe Psychiatry.
Source: School of Life Sciences
Editor: Li Mengjie
