The research team led by Professor Lu Yong of the School of Chemistry and Molecular Engineering of ECNU achieved a breakthrough in the research of efficient synthesis of methanol by carbon dioxide hydrogenation. The title of their work is “Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5towards efficient CO2hydrogenation to methanol”. It was published inScience Advances(2021, 7(32), eabi6012).

Meng Chao, a doctoral student, is the first author of the paper, and Professor Lu Yong and Associate Professor Zhao Guofeng of East China Normal University (ECNU) with Associate Professor Shi Xuerong of Shanghai University of Engineering and Technology are the paper’s co-correspondents. ECNU was granted the first completion unit.
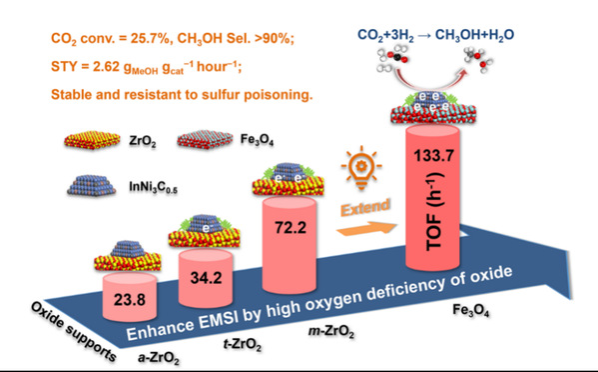
Direct CO2hydrogenation to methanol using renewable energy–generated hydrogen is attracting intensive attention, but qualifying catalysts represents a grand challenge.
For instance, the pure-/multi-metallic systems used for this task contain low catalytic activity. The researchers tailored a highly active and selective InNi3C0.5/ZrO2catalyst by tuning the performance-relevant electronic metal-support interaction (EMSI), which is closely linked with the ZrO2type–dependent oxygen deficiency. Highly oxygen-deficient monoclinic-ZrO2support imparts high electron density to InNi3C0.5because of the considerably enhanced EMSI, thereby enabling InNi3C0.5/monoclinic-ZrO2with an intrinsic activity three or two times as high as that of InNi3C0.5/amorphous-ZrO2or InNi3C0.5/tetragonal-ZrO2.
Moreover, the EMSI-governed catalysis observed in the InNi3C0.5/ZrO2system is extendable to other oxygen-deficient metal oxides, in particular InNi3C0.5/Fe3O4, achieving 25.7% CO2conversion with 90.2% methanol selectivity at 325oC, 6.0 MPa, 36,000 ml gcat−1hour−1, and H2/CO2= 10:1.
This affordable catalyst is stable for at least 500 hours and is also highly resistant to sulfur poisoning.
Paper Link: https://advances.sciencemag.org/content/7/32/eabi6012.full
Author: Xu Xincheng
Copy Editor: Joshua Mayfiled
Editor: Li Mengjie
